Vaccination for Respiratory Syncytial Virus: A Comprehensive Guide to Protection and Prevention
Respiratory Syncytial Virus (RSV) is one of the most common respiratory viruses worldwide, affecting people of all ages. While it often causes mild, cold-like symptoms in healthy adults and older children, RSV can lead to severe illness in vulnerable groups, including infants, older adults, and those with underlying health conditions. Each year, RSV is responsible for millions of infections, leading to significant hospitalizations and, in some cases, fatalities. Fortunately, recent advancements in medical research have led to the development of effective RSV vaccines and immunizations, offering new hope for preventing severe RSV disease.
In this article, we’ll explore everything you need to know about vaccination for respiratory syncytial virus, including how RSV spreads, who is at highest risk, the available RSV vaccines, their efficacy and safety profiles, current recommendations from health authorities like the CDC, and practical advice on getting vaccinated. Whether you’re a parent concerned about your baby’s health, an older adult looking to protect yourself, or simply seeking reliable information on RSV prevention, this guide provides evidence-based insights to help you make informed decisions.
Understanding Respiratory Syncytial Virus (RSV)
RSV is a seasonal virus that typically circulates during fall and winter months, peaking in colder weather. It spreads through respiratory droplets when an infected person coughs or sneezes, or via direct contact with contaminated surfaces. Symptoms usually appear 4-6 days after exposure and include runny nose, cough, fever, wheezing, and decreased appetite. In most cases, RSV resolves on its own within a week or two.
However, for high-risk individuals, RSV can progress to lower respiratory tract infections, such as bronchiolitis or pneumonia. According to the Centers for Disease Control and Prevention (CDC), RSV leads to approximately 58,000–80,000 hospitalizations among children under 5 years old and 100,000–160,000 among adults 65 and older annually in the United States. Globally, RSV is a leading cause of infant mortality in low- and middle-income countries.
Who Is at Risk for Severe RSV Infection?
Certain groups are more susceptible to severe RSV outcomes:
- Infants and Young Children: RSV is the leading cause of hospitalization in babies under 1 year. Premature infants, those with chronic lung disease, congenital heart conditions, or weakened immune systems face the highest risks.
- Older Adults: Adults aged 60 and older, especially those 75+, experience declining immunity, making RSV a significant threat. Conditions like heart disease, lung disease (e.g., COPD), diabetes, or kidney disorders increase vulnerability.
- Pregnant Individuals: While RSV rarely causes severe illness in healthy pregnant people, vaccinating during pregnancy protects newborns through passive antibody transfer.
- People with Compromised Immune Systems: This includes those undergoing chemotherapy, organ transplant recipients, or individuals with HIV.
Early recognition of symptoms and preventive measures are crucial for these groups.
Breakthroughs in RSV Vaccination: Available Options
After decades of research, 2023-2025 marked a turning point with the approval of multiple RSV prevention tools. There are three main RSV vaccines for adults and specific options for protecting infants.
RSV Vaccines for Older Adults
Three vaccines are approved for preventing RSV lower respiratory tract disease (LRTD) in adults:
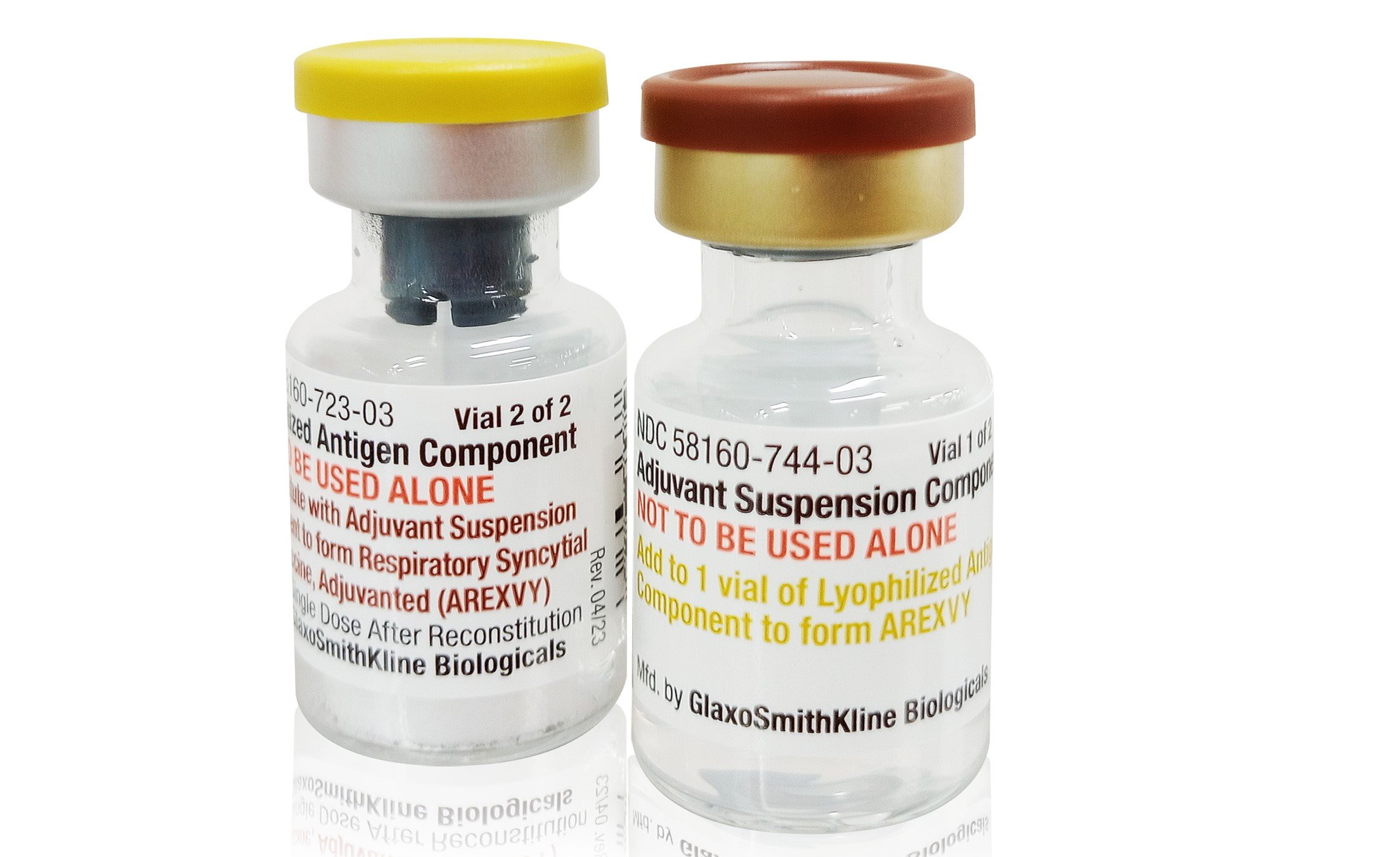
- Arexvy (GSK): A recombinant subunit vaccine containing the prefusion F protein with an adjuvant to boost immune response. Approved for adults 50-59 at increased risk and all adults 60+.
- Abrysvo (Pfizer): A bivalent prefusion F protein vaccine (no adjuvant). Approved for adults 18-59 at risk, 60+, and pregnant individuals.
- mResvia (Moderna): An mRNA-based vaccine, similar to COVID-19 vaccines, encoding the prefusion F protein.
These vaccines are administered as a single dose and have demonstrated strong efficacy. Clinical trials and real-world data show they reduce RSV-associated LRTD by 67-83% and hospitalizations by 60-80% over one to two seasons.
Protection for Infants and Young Children
Infants don’t receive traditional RSV vaccines due to historical safety concerns, but two approaches provide robust protection:
- Maternal Vaccination: Abrysvo is given to pregnant individuals between 32-36 weeks gestation (ideally September-January). Antibodies cross the placenta, protecting babies for up to 6 months. Efficacy against severe RSV in infants is 69-81%.
- Monoclonal Antibodies:
- Nirsevimab (Beyfortus): A single injection for infants entering their first RSV season or high-risk children in their second.
- Clesrovimab (Enflonsia): A newer option with similar protection, lasting at least 5 months.
These reduce severe RSV by 70-80%, dramatically lowering hospitalization risks. Palivizumab, an older monthly option, is being phased out by December 31, 2025.
Current Recommendations from the CDC (as of 2025)
The CDC provides clear guidance based on age and risk:
- Adults 75+: Recommended to receive one RSV vaccine dose.
- Adults 50-74 at Increased Risk: Recommended (conditions include lung/heart disease, diabetes, kidney failure, or severe obesity).
- Adults 60-74: Shared decision-making with providers.
- Pregnant Individuals: Abrysvo during 32-36 weeks to protect newborns.
- Infants: Maternal vaccination or monoclonal antibody (nirsevimab/clesrovimab) for all infants <8 months entering RSV season; extended for high-risk up to 19 months.
Vaccines are not annual yet protection lasts multiple seasons. Co-administration with flu or COVID-19 vaccines is safe.
Efficacy and Safety of RSV Vaccines
RSV vaccines have undergone rigorous testing:
- Efficacy: High against severe disease. Real-world studies confirm 77-83% effectiveness against hospitalizations over two seasons.
- Safety: Common side effects are mild: injection site pain, fatigue, headache, muscle pain, fever. These resolve quickly.
- Rare risks include Guillain-Barré Syndrome (GBS) in older adults (about 18 excess cases per million doses for some vaccines). Benefits far outweigh risks for recommended groups.
- For maternal/infant options: No significant preterm birth increase when timed correctly; serious adverse events balanced with placebo.
Ongoing monitoring by CDC and FDA ensures safety.
How to Get Vaccinated Against RSV
RSV vaccines are available at pharmacies, doctor’s offices, and clinics. Many insurance plans, including Medicare, cover them. Check with your provider or use vaccine finders on CDC.gov. Timing: Fall administration maximizes protection during peak season.
Frequently Asked Questions About RSV Vaccination
- Is the RSV vaccine safe during pregnancy? Yes, Abrysvo is specifically approved and recommended.
- Do I need it if I’ve had RSV before? Yesnatural immunity wanes.
- Can I get it with other vaccines? Yes, safely.
- What if I’m immunocompromised? Vaccination is especially important; consult your doctor.
Conclusion: Embracing RSV Prevention for a Healthier Future
Vaccination for respiratory syncytial virus represents a major public health victory. With options now available for older adults, pregnant individuals, and infants, we can significantly reduce the burden of severe RSV. Talk to your healthcare provider today about whether an RSV vaccine or immunization is right for you or your family. Staying informed and vaccinated is the best defense against this common yet potentially dangerous virus.




i like this post